LAPaL v2
Launched in 2021, the LAPaL project benefited this year from a major update that enhances its utility and scope. Developed on behalf of the Medicines Patent Pool (MPP) organisation, it aims to centralise and publicly present technical, scientific, and regulatory data on a selection of long-acting medical technologies. This interactive platform helps promote transparency, knowledge sharing, and equitable access to innovation in the field of global health.

Medicines Patent Pool
An important update was made this year to the LAPaL project, developed in 2021. This project, commissioned by the Medicines Patent Pool (MPP) organisation, aims to publicly present technical and scientific data on a selection of long-acting medical technologies. The information gathered in LAPaL primarily concerns the potential applications of these technologies as well as the intellectual property relating to them. The developments that have taken place since 2021 have been carried out in collaboration with Unitaid, the LEAP research programme, and the CELT research institute.
Updates
Since 2021, several updates have been made to the site, including the addition of the management of pharmaceutical compounds used as active ingredients in long-term medical technologies. These can be consulted in the same way as the technologies already displayed on the site, along with their links to long-term technologies. The LAPaL site has become a very comprehensive tool and allows for the management of all the data visible on the site through a fully customised management tool.
Comparison Tool
Comparative Mode & Export
From the main page of the site, it is now possible to compare the characteristics between compounds or between technologies. The user can select multiple items and then activate the comparison mode. This mode then presents each selected item in columns to visually compare their characteristics. The result can also be exported in an Excel format.

Statistics
This page provides an overview of the site's content and some statistics regarding the technologies recorded on the site. These statistics allow for comparisons of various characteristics of the technologies, notably the administration frequencies indicated for the uses of the technologies, or the therapeutic areas concerned with the technologies.
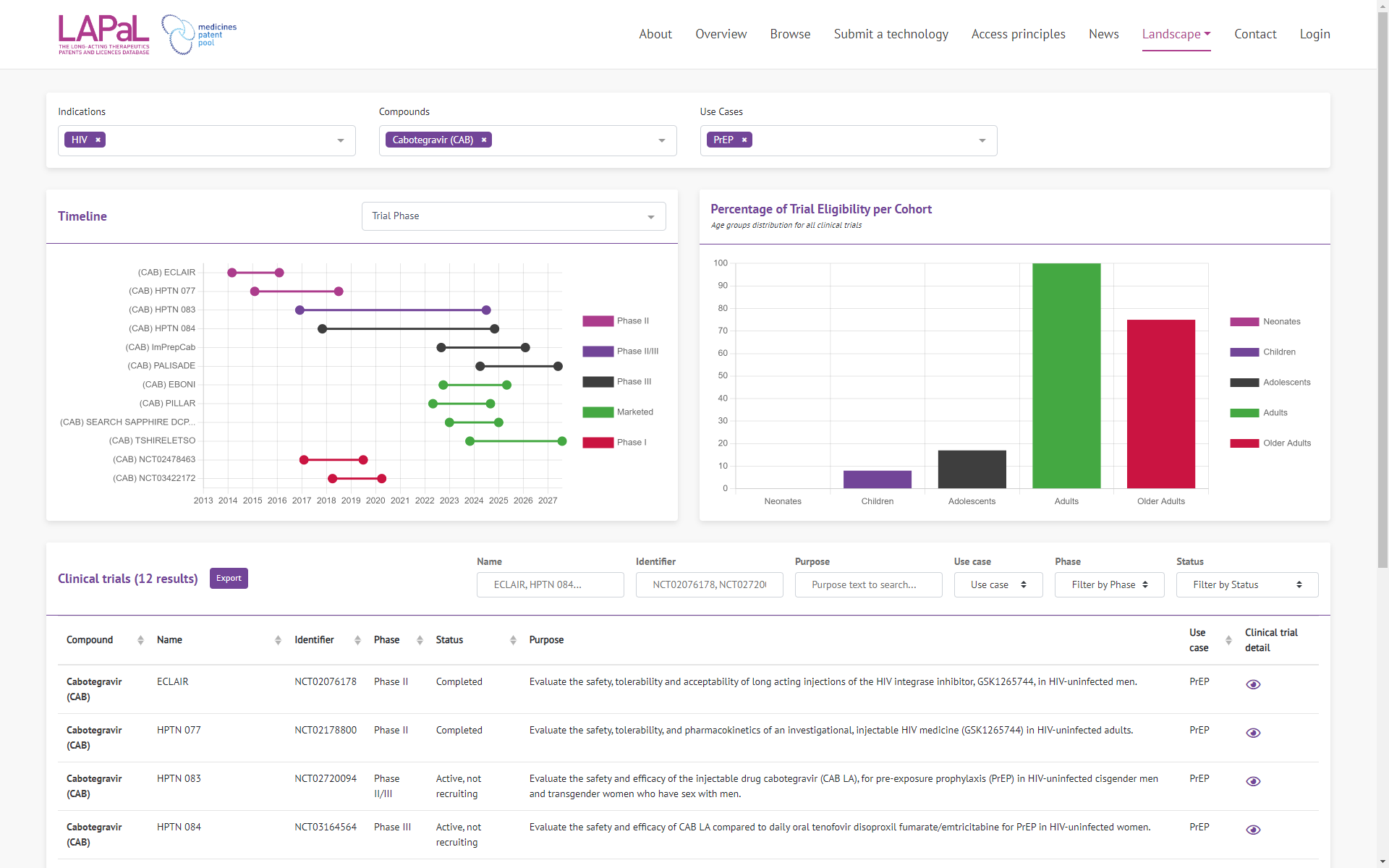
Clinical Trials
Clinical trials for both technologies and pharmaceutical compounds (referred to as “compounds” in LAPaL) were added in a previous update, but were presented summarily in the form of a table on the details pages. We have added a dedicated page for clinical trials to present, in graphical format, the clinical trials for one or more compounds/technologies. The first graph illustrates the active periods of these clinical trials in a timeline format, while the second presents the age ranges of the participants involved.
Import and Export
The data concerning the technologies and compounds is extensive, and entering this information can be time-consuming, particularly the data on clinical trials and the statuses of regulations. To facilitate the users responsible for content entry and to avoid input errors, we have implemented several data import tools from an Excel format. Similarly, we have added Excel exports containing all the information of a technology or a compound, as well as dedicated exports particularly for clinical trials and the statuses of regulations.
Whether for technologies or for compounds, the Excel files for imports contain around 120 fields spread across 8 Excel sheets. All content can be imported in this way, including the complete list of clinical trials and the statuses of regulations. The import tool was the most complex part of this new development, not only in terms of the volume of possible data but also regarding the necessary validations for each of these fields.
In conclusion
It is always a pleasure to work on the LAPaL project, which has continually evolved since its first version, both due to certain technical challenges and, more importantly, the richness of the relevant field. Developments will continue, particularly in consolidating the links between the technologies and registered compounds, as well as the possibility of creating composite elements from other already registered elements.
Acknowledgements
We would like to express our gratitude for the rich collaboration established since the outset of this project, as well as for the opportunity to engage with specialists in this field, which is both rich and complex, yet immensely fascinating.